According to Roothaan, it can be said that: By definition an electronic system possesses, in the ground state, a closed shell, if there is only one linear independent Slater determinant, which minimizes the energy expectation value within the SCF-HF method. It follows from this that such a system must fulfill the following conditions:
· The Hartree Fock ground state is NOT degenerate.
· The number of electrons is EVEN.
· Each spatial function is occupied by TWO electrons.
· The unique HF ground state wave
function ![]() (the over all
wave function)
(the over all
wave function)
represents a SINGLET function (![]() and
and ![]() ).
).
Assuming such a system while performing the SCF calculation, the term restricted Hartree Fock method (RHF) is applied and the Slater determinant is called a closed shell determinant. Molecules which can be sufficiently described by a closed shell determinant are called closed shell molecules.
Figure 2.1 Illustration of the orbital levels within the RHF and UHF picture. In the RHF method, for the a1 and b1 electrons, the same spatial function is used. In the UHF method, different spatial functions, j A and j C, are used. The orbital energy for the a 1 spin electron is stabilized and is lower in energy than the orbital of the b 1 electron. The spins of a1 and b1 do not cancel.
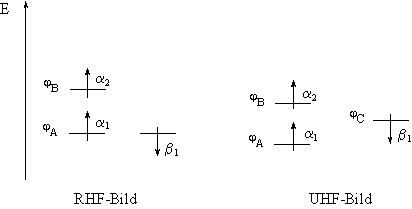
For excited states, or systems with an odd number of electrons (radicals),
the RHF method must fail as an open shell system must be used. Since
there are either more a or b
spin electrons, electrons in a doubly-occupied spatial function will interact
differently with the other (N-2) electrons. Here, different spatial functions
(no double occupation) are needed for an adequate description. Assuming
a system which contains an odd number of electrons, e.g. the orbital occupation ![]() (Figure
2.1), the following interaction have to be considered:
(Figure
2.1), the following interaction have to be considered:
1. Coulomb repulsion: a 1 spin electron with b 1 spin electron,
a 1 spin electron with a 2 spin electron
b 1 spin electron with a 2 spin electron
2. Exchange interaction: a 1 spin electron with a 2 spin electron.
Note that in contrast to the b spin electron
in ![]() , there is a stabilizing
exchange interaction of the a 1
spin electrons in
, there is a stabilizing
exchange interaction of the a 1
spin electrons in ![]() with
the a2 spin electron
in
with
the a2 spin electron
in ![]() . Through that, the
orbital energy of the a 1
spin electron is decreased (stabilized) and lies in an UHF (unrestricted
HF) calculation, in which for the a1
and b1 electrons, different
spatial functions will be used, lower than the orbital energy of the b1
electron. In this case, both spins (a1
and b 1) will not cancel
and it is referred to spin polarization.
. Through that, the
orbital energy of the a 1
spin electron is decreased (stabilized) and lies in an UHF (unrestricted
HF) calculation, in which for the a1
and b1 electrons, different
spatial functions will be used, lower than the orbital energy of the b1
electron. In this case, both spins (a1
and b 1) will not cancel
and it is referred to spin polarization.
If there is no special restriction for the orbitals in the UHF
(unrestricted Hartree Fock) method, e.g. the wave function is no
longer an eigenfunction of ![]() ,
then, analogous to the Roothaan equations, the matrix representation leads
to the Pople-Nesbet matrix equations, where there are two different sets
of molecular orbital expansion coefficients:
,
then, analogous to the Roothaan equations, the matrix representation leads
to the Pople-Nesbet matrix equations, where there are two different sets
of molecular orbital expansion coefficients:
F(a )c(a ) = e(a )S(a )c(a )
and
F(b )c(b ) = e(b )S(b )c(b ).
Both equations are coupled via the Fock operator, ![]() ,
and hence represent two non-independent, generalized matrix-eigenvalue
problems.
,
and hence represent two non-independent, generalized matrix-eigenvalue
problems.
One major disadvantage is that the wave functions are not pure spin
states (i.e. there is no eigenfunction of ![]() ),
but contain some spin contamination from higher states, e.g.
·
N3 radical should have:
),
but contain some spin contamination from higher states, e.g.
·
N3 radical should have: ![]() =
0.75 [S(S+1)] but at UMP2/6-31G(d) level,
=
0.75 [S(S+1)] but at UMP2/6-31G(d) level, ![]() =
0.904 is calculated.
=
0.904 is calculated.
![]()
Such a large spin contamination corresponds to a sizable influence of
quartet spin states on the doublet. Usually this not a serious difficulty,
since the spin contamination is often relatively small. For example, ![]() =
0.7623, 0.7575, 0.7591 for · C2H5,
·
CH3O. and · CH2OH,
respectively, at UHF/6-31G(d) level. 0.75 is the value obtained for a pure
doublet.
=
0.7623, 0.7575, 0.7591 for · C2H5,
·
CH3O. and · CH2OH,
respectively, at UHF/6-31G(d) level. 0.75 is the value obtained for a pure
doublet.
Two approaches can be used to solve the UHF spin-contamination problem. Either the calculation is began with a pure spin state such as an ROHF (restricted open Hartree Fock) wave function, or the spin-contamination in the UHF function must be annihilated.
______________________
* The expectations value of ![]() indicates
the extent to which the UHF process has produced a mixture of different
spin states.
indicates
the extent to which the UHF process has produced a mixture of different
spin states.
______________________
Like the RHF wave function, the ROHF wave function is eigenfunction of both the spin operators S2 and Sz. In the ROHF procedure, one part of the orbitals will be doubly occupied (i. e. restricted, e.g. the core electrons), the other part however, will be singly occupied (valence electrons).
In order to give more reliable estimates of the reaction energies involving open shell species, techniques of spin projection can be used, which eliminate some (but not all) contributions from higher states.
last changes: 01.04.2008, AS questions & comments to: axel.schulz@uni-rostock.de