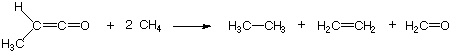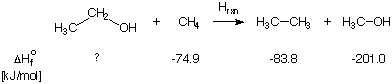Isodesmic Reactions
Isodesmic reactions are defined (see comments in W. J. Hehre, R. Ditchfield, L. Radom, J. A. Pople,
J. Am. Chem. Soc. 1970, 92, 4796) as
" . . chemical changes in which there is retention of the number of bonds
of a given formal type,
but with a change in their relation to one another".
Isodesmic reactions represent a subclass of isogyric reactions,
the latter of which are defined as transformations in which reactants and products have the same number of
electron pairs. These reactions can, in principle, be purely hypothetical. To illustrate the concept with one of the
original examples, let us consider the reaction of methyl ketene with methane:
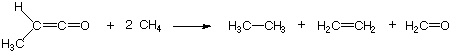
On the reactant as well as the product side one can identify the following bond types:
1 C-C single bond, 1 C-C double bond, 12 C-H single bonds, 1 C-O double bond. This is also
an example of a bond separation reaction, in which the bonds between
non-hydrogen atoms are cleaved such that the simple most diatomic molecules are generated.
Due to the constant number of bonds of a given type, the reaction energies of isodesmic reactions
can be predicted quite accurately already with relatively cheap theoretical methods.
This property makes isodesmic reactions a valuable tool for the prediction of the heat of formation of
unknown organic compounds. In order to illustrate the procedure, let us consider the reaction of
ethanol with methane:
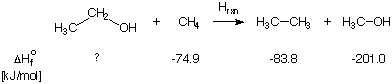
After accounting for all different bond types involved in this reaction equation, it is clear that
this reaction does classify as an isodesmic reaction. The (standard state) heats of formation
for three of the four species are known to good accuracy and are given below the equation. The heat of formation
of the fourth species (ethanol) can be estimated now by simply calculating the reaction energy ERXN
(or better: the reaction enthalpy HRXN), with quantum mechanical methods and by using the
computed reaction energy together with the known heats of formation to estimate that of ethanol:
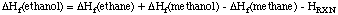
Using cheaply calculated HF/STO-3G energies for all four species, a reaction energy of +10.9 kJ/mol is
predicted. Together with the know heats of formation, a value of -220 kJ/mol is predicted for ethanol.
This has to be compared to the experimental value of -235.3 kJ/mol.
The success of this procedure strongly depends on the quality of the available heats of formation.
It should also be remembered that different isodesmic reactions can be set up in order to estimate
the heat of formation of one and the same species. Different isodesmic reactions will give different
results without one being systematically better than the other. In case the heats of formation of all
involved species are known to good accuray, isodesmic reactions can also be used to validate theoretical
methods.
Isodesmic reactions can also be used to quantify the stability of reactive
intermediates in a thermochemical sense. If, for example, we want to quantify the stability of the
methanol-1-yl radical, we could simply use the bond dissociation energy of the methanol C-H bonds. This is
synonymous to calculating the reaction energy for the following reaction:
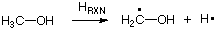
While the energy of this reaction will without question reflect the stability of the two product radicals,
theoretical prediction is by no means straight forward. The electron correlation energies will be
substantially different in the closed shell reactant and in the two open shell products, requiring a sophisticated
treatment of electron correlation effects. Using an appropriate isodesmic reaction such as
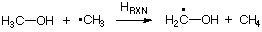
simplifies the situation considerably. This hydrogen transfer reaction between methanol
and methyl radical can be constructed as the difference between the C-H bond dissociation reactions
in methane and in methanol. The reaction energy of this isodesmic reaction therefore reflects the
difference in C-H bond dissociation energies in methane and methanol and thus the differential stability
of the methyl and the methanol radical. Using experimentally determined heats of formation at 298.15K
of -17.8 kJ/mol (methanol radical), -74.87 kJ/mol (methane), -201.1 kJ/mol (methanol), and +145.69 kJ/mol
(methyl radical) one arrives at an isodesmic heat of reaction of -37.3 kJ/mol. This implies that the
methanol radical is substantially more stable than the methyl radical. Similar considerations lead to
a gain in radical stability of -17.8 kJ/mol on going from the methyl to the ethyl radical.
last changes: 01.04.2008, AS
questions & comments to: axel.schulz@uni-rostock.de