Part A
Calculate for H+, H, H-, H-H+ (d = 1.03 A) and H-H (d = 0.74 A) the nucleus - nucleus repulsion energy, the nucleus - electron attraction energy, the kinetic energy of the electron and the total energy at HF/STO -5G level! Discuss the results and find a connection between the different energies to be considered.
| H+ | H | H- | H-H+ | H-H | |
| d/A | - | - | - | 1.03 | 0.74 |
| charge/multiciplicity | 1,- | 0,2 | -1,1 | 1,2 | 0,1 |
| E(KK)/a.u. | - | 0.0 | 0.0 | +0.5137643194175 | 0.7151043905405 |
| E(K-e)/a.u. | - | -1.23873697134 | -2.47747394268 | -1.687217914282 | -3.723518053615 |
| E(e)/a.u. | - | +0.767994053078 | +1.535988106155 | +0.5875400375793 | 1.208886785438 |
| E(HF)/a.u. | - | -0.4707429 | -0.1664941 | -0.5859136 | -1.1248243 |
Discussion
E(KK) = nucleus - nucleus repulsion
E(K-e) = nucleus - electron attraction
E(e) = kinetic energy of the electron
For all one-electron systems: E(HF) = E(KK) + E(K-e) + E(e)
e.g.
E(HF, H) = E(K-e) + E(e) = -1.23873697134 + 0.767994053078 = -0.4707429 a.u.
and
E(HF, H-H+) = E(KK) + E(K-e) + E(e) = +0.5137643194175 - 1.687217914282 + 0.5875400375793 = -0.5859136
For multi-electron systems E(e-e) and E(exchange) have to be considered, additionally:
E(HF) = E(KK) + E(K-e) + E(e) + E(e-e) + E(x)
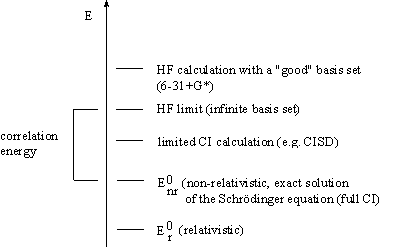
The calulated nonrelativistic Hartree Fock limit is always above the exact energy. The difference
E(exact,nonrel.) - E(HF,limit) = E(correlation)
is called correlation energy.
Part B
Post-HF Methods - Explicit Consideration of the electron correlation:
Calculate for the H-H molecule (d = 0.74 A) the energies at HF, MP2, MP3, MP4 ab initio level as well using density functional theory at the BHLYP, B3LYP and BLYP level!
| HF | MP2 | MP3 | MP4(SDTQ) | |
| E(total)/a.u. | -1.1248243 | -1.1379921 | -1.1428378 | -1.1445518 |
| BHLYP | BLYP | B3LYP | ||
| E(total)/a.u. | -1.1248243 | -1.1632733 | -1.1634565 | -1.1738267 |
Discussion

Introduction of the electron correlation energy decreases the total
energy. The HF energies at all levels of theory possesses the same values.
Part C
Optimize the geometry of H-H at HF and MP2 level. Compare and discuss the geometry and energies!
| H-H distance | E(HF)/a.u. | E(MP2)/a.u. | |
| HF | 0.71070543 | -1.1256574 | - |
| MP2 | 0.72215245 | -1.1255257 | -1.1382817 |
Discussion
The HF energy at HF and MP2 level are different as the geometry at both
levels is different. Since the MP2 distance does not represent the HF minimum
the MP2-HF energy is lower. The H-H distance is longer at MP2 level due
to electron correlation (occupation of antibonding orbitals resulting in
larger distances). Neglecting electron correlation often results in too
short bond lengths.
last changes: 01.04.2008, AS questions & comments to: axel.schulz@uni-rostock.de