Stabilization of classical anhydrous Brønsted acids
Poster presentation by Kevin Bläsing
Kevin Bläsing, Jonas Bresien, Lukas Chojetzki, Henrik Müller, Axel Schulz, Alexander Villinger, and Ronald Wustrack
Abstract
Hydrogen pseudohalides:[1] Until now, there are only a few investigations addressing the stabilization of classical, unstable hydrogen pseudohalides (HX, X = pseudohalogen). For example, the stabilization with bulky Lewis acids by adduct formation can give access to such labile systems. Recent results of our group revealed that it was possible to isolate and characterize a series of Lewis-acid Brønsted-acid adducts.[2] For example, the first hydrogen azide adduct and a dimer of hydrogen cyanide could be isolated.
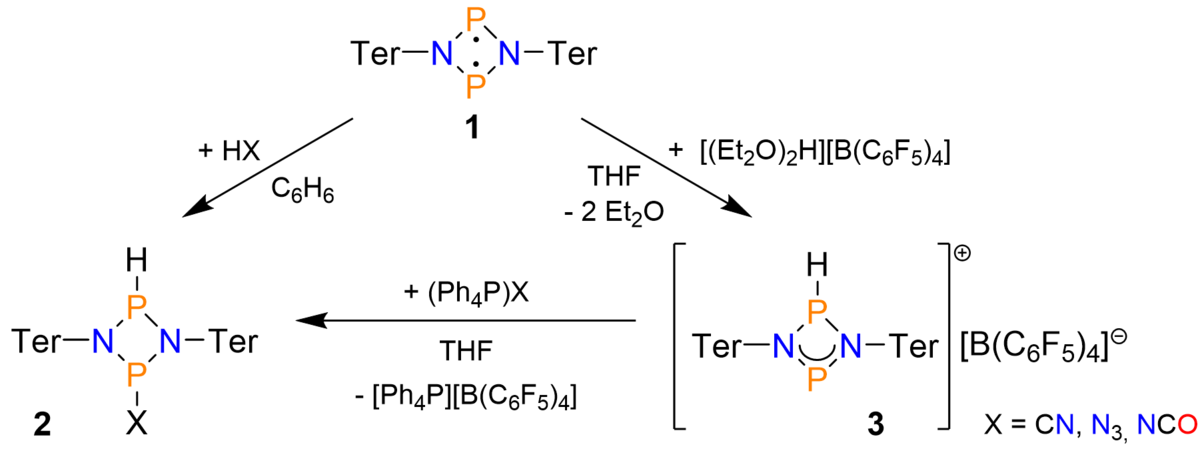
As biradicals are known to activate highly labile compounds,[3] we were interested in the adduct formation between group 15 biradicals (e.g. [P(µ-NTer)]2, 1; Scheme 1) and hydrogen pseudohalides. Indeed, as shown in Scheme 1, it was possible to activate a series of different Brønsted acids (HX, X = CN, N3, NCO) by direct treatment of the corresponding acid with the biradical (cf. 2). Alternatively, the biradical-HX adducts could also be obtained in a two-step procedure by protonating the biradical with Jutzi’s acid (cf. 3), followed by treatment with a “naked” pseudohalide (e.g. [Ph4P]X).
Scheme 1. Reactivity of biradical [P(µ-NTer)]2 with Brønsted acids.
References
[1] Birkenbach L., Kellermann K. Über Pseudohalogene Ber. Dtsch. Chem. Ges. B 1925, 58, 786−794.
[2] a) Labbow R., Michalik D., Reiß F., Schulz A., Villinger A. Isolation of Labile Pseudohalogen NSO Species Angew. Chem. Int. Ed. 2016, 55, 7680−7684; b) Bläsing K., Bresien J., Labbow R., Schulz A., Villinger A. A Dimer of Hydrogen Cyanide Stabilized by a Lewis Acid Angew. Chem. Int. Ed. 2018, 57, 9170−9175; c) Bläsing K., Bresien J., Labbow R., Michalik R., Schulz A., Villinger A. Borane Adducts of Hydrazoic Acid and Organic Azides: Intermediates for the Formation of Aminoboranes Angew. Chem. Int. Ed. 2019, 58, 6540−6544.
[3] Abe M. Diradicals Chem. Rev. 2013, 113, 7011-7088.