Experimental and Theoretical Aspects of Group 15 Biradicals [E(µ-NBbp)]2 (E = P, As)
Poster presentation by Lilian Sophie Szych
Lilian Sophie Szych, Jonas Bresien,Alexander Villinger, Ronald Wustrack and Axel Schulz
Abstract
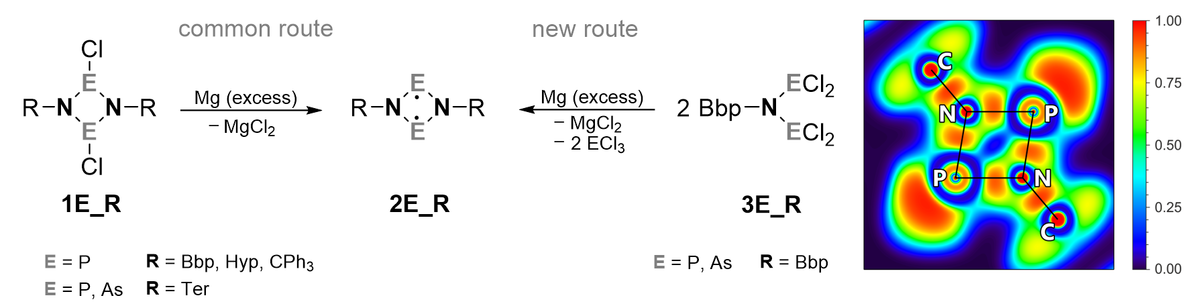
Group 15 open shell singlet biradicals[1a,b] of the type [E(µ-NR)]2 (E = P, As) show an interesting and diverse reaction behaviour, for example in the activation of molecules bearing multiple bonds. Commonly, R is a sterically demanding substituent which ensures kinetic stabilization of the highly reactive biradical.[2-4] The synthesis of new biradicals stabilized by the Bbp substituent (2,6-bis[bis(trimethylsilyl)methyl]phenyl)[5] was investigated: Reducing 1E_Bbp (E = P)via the “common route”, it is possible to synthesize the desired biradical 2E_Bbp, reduction of the doubly substituted acyclic compounds 3E_Bbp (E = P, As) also led to the corresponding biradicals, which represents a hitherto unknown route to this class of compounds (Scheme 1).
The synthesised biradicals 2E_Bbp were studied theoretically regarding their biradical character and the bonding situation using CASSCF and MRCI calculations. Furthermore, the topology of the electron density was evaluated to gain insight into the bonding situation using the quantum theory of atoms in molecules (QT-AIM). The results of the detailed analysis of such biradicals showed that the steric hindrance and the electron transfer from the substituent to the 4-memebered ring have an influence on the target structure. They also confirm the biradical character of these systems and do not indicate a transannular bonding interaction between the heavier pnictogen atoms of the biradicals.
Scheme 1. Two reduction paths leading to biradicals 2E_R (left) and ELF of 2P_Bbp (right).
References
[1] a) M. Abe, Chem. Rev. 2013, 113, 7011–7088.; b) F. Breher, Coord. Chem. Rev. 2007, 251, 1007–1043.
[2] T. Beweries, R. Kuzora, U. Rosenthal, A. Schulz, A. Villinger, Angew. Chem. Int. Ed. 2011, 50, 8974–8978.
[3] A. Hinz, R. Kuzora, A. Rölke, A. Schulz, A. Villinger, R. Wustrack, Eur. J. Inorg. Chem. 2016, 22, 3611–3619.
[4] S. Demeshko, C. Godemann, R. Kuzora, A. Schulz, A. Villinger, Angew.Chem. Int. Ed. 2013, 52, 2105–2108.
[5] T. Agou, Y. Sugiyama, T. Sasamori, H. Sakai, Y. Furukawa, N. Takagi, J. Guo, S. Nagase, D. Hashizume, N. Tokitoh, J. Am. Chem. Soc. 2012, 134, 4120–4123.