Small molecule activation with open shell singlet biradicaloids
Biradicals and biradicaloids are highly reactive species that can occur in the processes of bond formation and bond breaking. They were discussed as intermediates even in Diels-Alder reactions. Hence, the study of biradicaloids is of general importance.
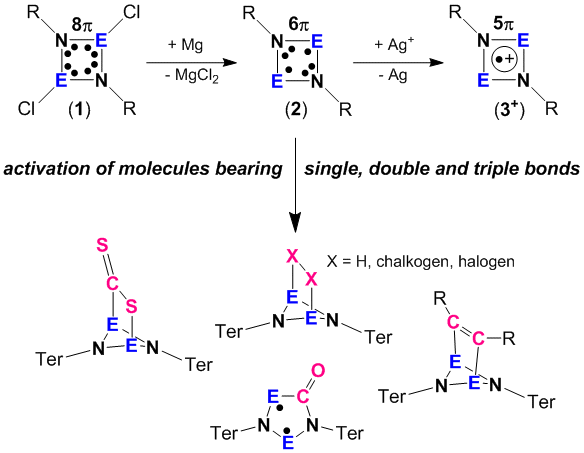
Our research in this field deals with the synthesis and full characterization of biradicaloids of the type [E(m-NTer)]2 (E = element of group 15, Scheme 1 species 2).[1-3] The reactivity of these biradicaloids 2 was employed to activate small molecules bearing single, double and triple bonds. Addition of chalcogens (O2, S8, Sex and Tex) led to the formation of dichalcogen bridged E2N2 heterocycles. In formal [2+2] addition reactions small unsaturated compounds such as ethylene, acetylene, acetone, acetonitrile, tolane, diphenylcarbodiimide, and bis(trimethylsilyl)¬sulfurdiimide are re-adily added to the E2N2 heterocycle of the biradicaloid 2 yielding novel heteroatom cage compounds. The reaction with CO and isonitriles led to the formation of new cyclic 5-membered heterocycles featuring also biradical character. Oxidation with silver salts gave stable cyclic radical cations (3+).
Group 15 open shell singlet biradicaloids such as our [P(µ-NTer)]2 (2) can be utilized in the activation of stable small molecules. For example, fast reactions with H2, CO2, and NH3 were observed. Dihydrogen easily adds to [P(µ-NTer)]2 yielding [HP(µ-NTer)]2 at ambient conditions while the reversible release of molecular hydrogen was found at slightly elevated temperatures (T > 60°C).
[1] A. Hinz, R. Kuzora, A. Schulz, A. Villinger, Chem. Eur. J. 2014, 20, 14659 – 16673.
[2] A. Hinz, A. Schulz, A. Villinger, Angew. Chem. Int. Ed. 2015, 54, 668 – 672.
[3] A. Hinz, A. Schulz, A. Villinger, Angew. Chem. Int. Ed.2015, 54, 2776 – 2779.