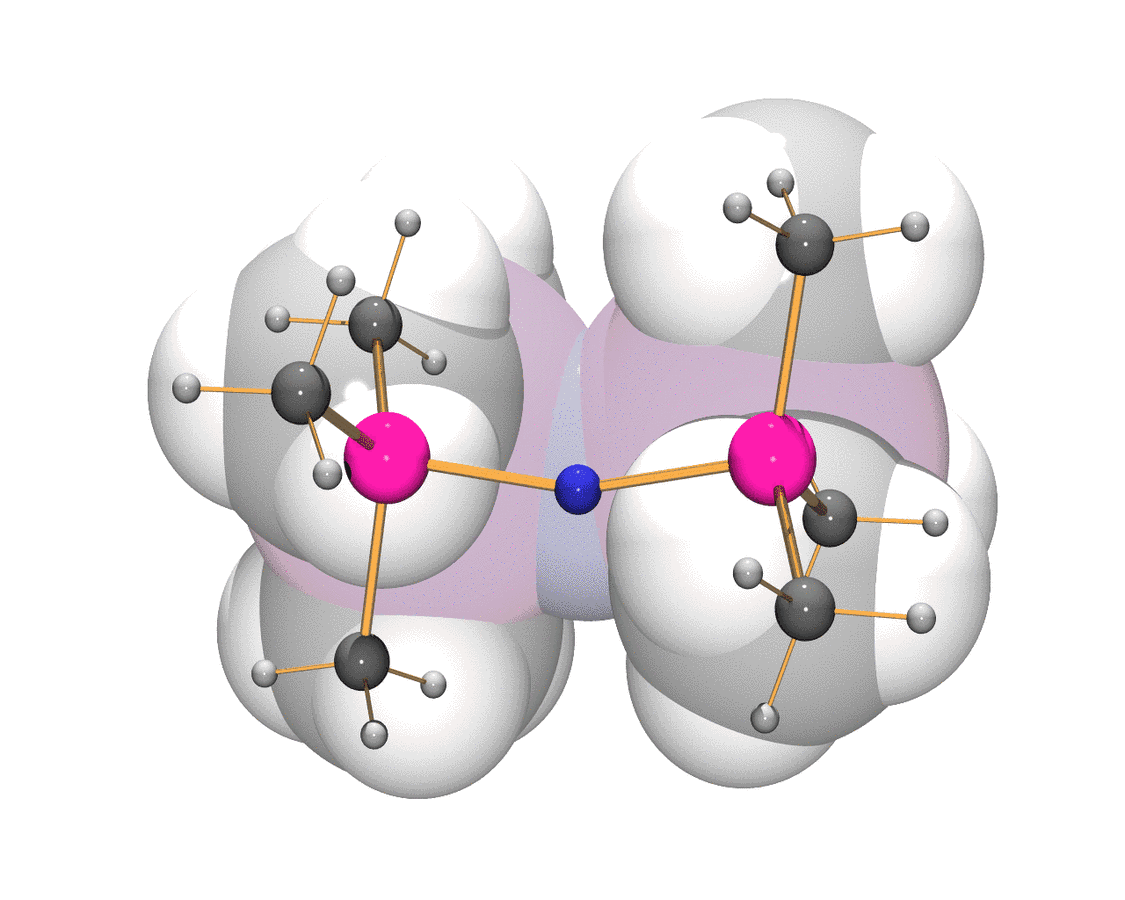Halogen - Pseudohalogen Chemistry
Part of our reseach with weakly coordinating anions is their utilization to stabilize reactive cations such as halonium and pseudohalonium ions. Since it is possible to isolate halonium species of the type R-X-R+ (X = F, Cl, Br, I) it was to prove if this is also possible for the so-called pseudohalogens. And by doing this to prove and extent the pseudohalogen concept, which provides a powerful tool to understand the correlation between chemical properties, structure and bonding of pseudohalogen species.
The term pseudohalogen was first introduced by Lothar Birckenbach in 1925. This concept of pseudohalogens includes severel criteria of classical pseudohalogens with respect to a halogen-like chemical behaviour. A pseudohalogen (X) forms
- a strongly bound (linear) univalent radical (X×),
- a singly charged anion (X–),
- a pseudohalogenhydrogen acid of the type HX,
- salts of the type M(X)n with silver, lead and mercuric salts of low solubility,
- a neutral dipseudohalogen compound (X–X) which disproportionates in water and can be added to double bonds, and
- interpseudohalogen species (X–Y).
But not all criteria are always met. While many linear pseudohalogens (e.g. CN, OCN, CNO, N3, SCN) are known, often the corresponding pseudohalide acids, dipseudohalogens, and interpseudohalogens are thermodynamically highly unstable (e.g. HN3, OCN–NCO, NC–SCN) with respect to N2/CO elimination or polymerisation or indeed, remain unknown (e.g. N3–N3).
Starting from the binary non-metal hydrides CH4, NH3, H2O etc. a simple approach can be utilized to derive the hydrogen acids of the classical linear pseudohalides by isolobal, isosteric or isoelectronic combination or substitution. The nonlinear resonance-stabilizedpseudohalides can be derived with the help of the Grimm hydride displacement law, a pseudoelement concept, which describes the formation of a new pseudoelement ×AHn when nH (n = 1 – 4) atoms are formally added to the element A. The new complexes AHn (e.g. OH, NH2 and CH3) behave like pseudoatoms (in this case like halogens) similar to elements of the group n positions to the right in the periodic table. However, chemically there is a significant difference in the basicity between these pseudohalides and the halides. Only the successive substitution of the hydrogen atoms in CH3 (CH3–) and NH2 (NH2–) by electron-withdrawing groups such as CN, NO, and NO2, leads to the class of resonance-stabilized, nonlinear pseudohalogens (pseudohalides). Important is also the capability of the electron-withdrawing groups to delocalize the single p-AO-type lone pair (AO = atomic orbital) of the C and N atom in CH3 (CH3–) and NH2 (NH2–), respectively, to decrease the basicity into the range of the halides.
The pseudohalogen concept can be extended
- to some special non-planar anions such as CF3–,
- to derivatives such as the five-membered ring anion [CS2N3]–,
- to heavier elements, which results in weaker delocalization effects due to the formation of weaker pi-bonds.
Pseudohalonium ions
Ions of the type [R–X–R]+, where X is any halogen are referred to as halonium ions which may be open-chain or cyclic, and are an important class of onium ions. They play a major role in preparative chemistry as reaction intermediates e.g. in Friedel-Crafts alkylation or Lewis acid catalyzed halogenation reactions.Dialkyl chloro-, bromo-, and iodonium ions can be prepared as stable long-lived ions and even isolated as stable salts. However, no stable dialkylfluoronium ion has been obtained in the condensed phase which has been attributed to the high electronegativity leading to a C–H (or C–C) bond alkylation. As early as 1988 the [H–F–H]+ ion was observed in [H–F–H][Sb2F11] (= 2 HF∙SbF5), which was isolated from the superacidic medium HF/SbF5. The bulky trimethylsilicenium ion [Me3Si]+ may be regarded as a sterically demanding “big proton”. Hence, similar to a proton which is always associated in the condensed state, e.g. in anhydrous HF(l) it forms [H–F–H]+ or it protonates HSO3F(l) to [H2SO3F]+, it can be assumed that [Me3Si]+ is also always solvated and silylates the solvent forming the corresponding analogous cations. Thus, the idea was to react [Me3Si]+-salts with trimethylhalogenosilanes Me3Si–X (X = F, Cl, Br, and I) to give bis(trimethlsilyl)halonium cations [Me3Si–X–SiMe3]+, where Me3Si–X is both solvent and reactant. [Me3Si]+-salts are only stable in the presence of chemically robust, weakly coordinating anions such as carborate ions (e.g. [HCB11F11]–) or tetrakis(pentafluorophenyl)borate, [B(C6F5)4]–. We carried out the synthesis and full characterization of the first acyclic fluoronium cation [Me3Si–F–SiMe3]+ as well as on the hitherto unknown analogous chloronium, bromonium, and iodonium cations, providing a complete set of the same type of halonium ions which have been fully characterized as tetrakis(pentafluorophenyl)borate salts.
Pseudohalonium ions
By utilizing reaction mixtures such as Me3Si–X/[Me3Si–X–SiMe3]+ (X = CN, OCN, SCN, and NNN), it was possible to prepare the first examples of bissilylated pseudohalonium cations. Salts containing pseudohalonium cations are only stable in the presence of weakly coordinating anions such as the well-known tetrakis(pentafluorophenyl)borate, [B(C6F5)4]–.
In regard to the pseudohalogen concept we have shown in this study that this concept can be extended by the criterion that a pseudohalogen should also form a pseudohalonium ion e.g. of the type [Me3Si–X–SiMe3]+.