Pnictogene Chemistry
Our group is especially interested in
- CN
- EN (E = element of group 15)
- SN
chemistry with the focus on binary cyclic and acyclic species which can be either ions (cations or anions) or neutral systems. To achieve this goal we have developed a new [3+2] cycloaddition reaction which utilizes disguised 1,3 dipoles and/or dipolarophiles. Moreover, our research is focussed on small molecule activation by utilizing four-membered E2N2 biradicaloids developed in our group.
Kinetic Protection – Bulky Groups
Since binary EN compounds are mostly thermodynamically unstable species, kinetic protection is needed to stabilize binary EN compounds. Here we use well-established bulky groups such as the terphenyl, supermesityl or hypersilyl group.
Stabilization of Strong Electrophiles – Weakly Coordinating Ions
In case of binary EN ions, the counterion must fullfil two criterions:
- It must be chemically inert.
- It should be weakly coordinating.
CN-chemistry
- major goal: synthesis and characterization of tricyano-amine, N(CN)3 – so far not succeed
- synthesis of new pseudohalogen compounds which can be obtained either by nucleophilic attack or in a multi-step synthesis
- we were able to isolate several mono-, di- and tri-substituted cyanuric species, amides and methanides of the type: [NR1R2]– and [CR1R2R3]– with R1,2,3 = H, CN, NO, NO2, CHO (non-linear resonance stabilized pseudohalides)
- new binary molecular, oligomeric and polymeric CN species are of special interest
- first structural report of neutral mixed crystals of phenylcyanamide
- we will try to introduce the resonance-stabilized pseudohalides e.g. dicyanamide into CN rings, respectively, since valuable precursor for polymerisation are expected to be obtained
NN-chemistry
- highly labile salt bearing the trissilylated homoleptic diazenium ion [(Me3Si)2N=N-SiMe3]+ was prepared
- direct silylation of Me3Si-N=N-SiMe3 failed due to side reactions or low solubility
- straightforward two-electron oxidation process starting from mercury(II) bishydrazide and Ag[GaCl4]
PN-chemistry
Neutrals rings
- only a very small number of planar five-membered ring molecules and ions containing only the elements phosphorus(III) and/or nitrogen have been isolated
- synthesis of TMS-PNN or TMS-NNP species
- formation of the first binary neutral PN ring: the 4-bis(trimethyl)amino-1,2,4,3,5-triazadiphosphole
- using [3+2] cycloaddition reactions we are interested in the synthesis of new binary NxPy (x + y = 5) heterocycles stabilized as adducts
Cationic rings
- binary cyclic P(III)/N four-membered heterocyclic cations, with a di- and tricoordinated P atom and a delocalized p bond along the NP(+)N unit
Phosphorus azides
- GaCl3 assisted [3+2] cycloadditionwhich led to the isolation of the first binary phosphorus/nitrogen
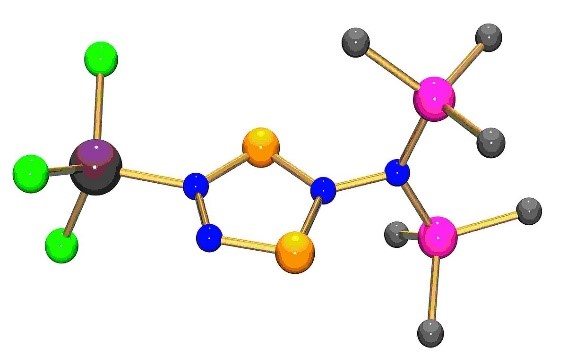
- access to tetrazaphospholes and -arsoles was gained by two different synthetic approaches: reaction of a diazane with Me3Si-N3 or reaction of Me3Si-N3 and Mes*N(SiMe3)PCl2
- both approaches are triggered by addition of Lewis acid GaCl3
- the reactive species is generated in situ by GaCl3-assisted elimination of Me3Si–Cl
AsN-chemistry (Tetrazaarsole)
- concept of GaCl3-assisted formal [3+2] cycloaddition
- isolation of the first binary arsenic/nitrogen five-membered heterocycle, a tetrazaarsole
- investigation of the equilibrium between cyclic diarsadiazane and its monomer, the iminoarsane
- addition of one equivalent of Me3Si–N3 followed by one equivalent of GaCl3 leads to disappearance of the red color of the monomer and formation of one new colorless species
BiN-chemistry
- in the case of bismuth, only a few neutral trivalent examples of homoleptic Bi–N compounds and to the best of our knowledge only one cationic di-coordinated cyclic Bi–N species are
- one straightforward approach applied to stabilize heavy element double bonds is the strategy of introduction of bulky groups
- low coordination and a positive charge can be realized by delocalization and stabilization by means of bases (charge transfer
- synthesis and isolation of binary bismuth(III) azides and their full characterization
- the structural elucidation of [Bi(III)(N3)4]–- and [Bi(III)(N3)6]3–-salts fills an open gap in main group chemistry
- Bi(N3)3·THF could qualify as a precursor for the generation of binary BiN in detonation experiments due to its low shock and heat sensitivity
- first acyclic homoleptic di-coordinated bismuth-nitrogen cation with a Bi–N double bond due to silylated hydrzino groups
- the cation can also be regarded as a new member of the class of nitrogen rich pentapnictogenium cations of the type R6E5+
SN-chemistry
- synthesis of new binary and ternary SN and NSCl compounds (oligomers, polymers and radicals)
- we succeeded to isolate and fully characterize the first aza analogue of thionyl dichloride – the naked NSCl2–-anion
- we could show, that the heavier the halogen the less stable are these anions and decompose yielding (SN)x and SNX (X = Br or I) polymers
- the investigation of the donor-acceptor properties of NSCl resulted in a surprising synthesis of the hitherto unknown S2N3+ cation representing the first binary Hückel aromatic system with an N3 unit
- we will try to replace the halogens by pseudohalogens and to synthesize polymers with dithiadiazolium groups
- one-electron reduction should result in the formation of paramagnetic polymers which could qualify as new molecular magnets
- the C5H5– analogue S2N3+ should be introduced into metal organic complexes
Biradicals and biradicaloids are highly reactive species that can occur in the processes of bond formation and bond breaking. They were discussed as intermediates even in Diels-Alder reactions.
Our research in this field deals with the synthesis and full characterization of biradicaloids of the type [E(m-NTer)]2 (E = element of group 15, species 1). The reactivity of these biradicaloids 1 was employed to activate small molecules bearing single, double and triple bonds. In formal [2+2] addition reactions small unsaturated compounds such as ethylene, acetylene, acetone, acetonitrile and other are re-adily added to the E2N2 heterocycle of the biradicaloid 1 yielding novel heteroatom cage compounds. The reaction with CO and isonitriles led to the formation of new cyclic 5-membered heterocycles featuring also biradical character. Oxidation with silver salts gave stable cyclic radical cations (2+).
Group 15 open shell singlet biradicaloids such as our [P(µ-NTer)]2 (1) can be utilized in the activation of stable small molecules. For example, fast reactions with H2, CO2, and NH3 were observed. Dihydrogen easily adds to [P(µ-NTer)]2 yielding [HP(µ-NTer)]2 at ambient conditions while the reversible release of molecular hydrogen was found at slightly elevated temperatures (T > 60°C).
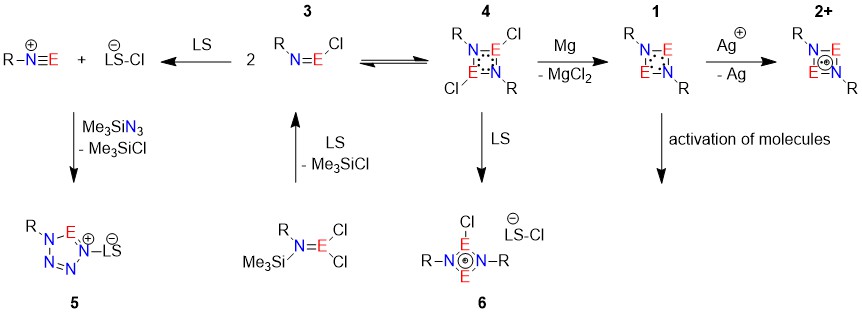
The equilibrium of the monomer RN=PCl (3) and its dimer 4 depends on steric influence of the substituent. It is also starting point of the synthesis of cyclic tetrazaphospholes (E = P, 5) by Lewis acid assisted chloride abstraction and [3+2] cycloaddition or cyclic 4-membered cations (6) using strong Lewis acids.
Especially, we focus on the synthesis of new cyclophosphanes using different P1-bulding blocks like mono-substituted phosphanes or phosphorus trichloride. An exergonic process of isomerization leads to cyclo-tetraphosphanes being capable of manifold chemistry, for example reactions with Lewis acids and bases and [4+2] cycloadditions.